AG Meierjohann
Tumor biochemistry
Stress defence and phenotypic reprogramming in melanoma
Melanoma belongs to the most frequent malignant tumor entities in Europe. Despite the availability of kinase inhibitors and immune therapy, melanomas frequently evade therapeutic pressure, and improved treatment strategies are needed.
Our group investigates the tumor adaptation processes in defence to stress, which melanoma cells encounter e.g. in the tumor niche or after exposure to therapeutics.
The antioxidant transcription factor NRF2 and the integrated stress response effector ATF4 represent two acute stress mediators that reprogram melanoma cells in response to these stress triggers, thereby increasing melanoma resilience and therapy resistance. Due to their important role in thiol metabolism, they are tightly connected to ferroptosis, a form of cell death initiated by iron-mediated lipid peroxidation. Using a combination of functional and interventional approaches, we analyse the roles of NRF2 and ATF4 in melanoma reprogramming with particular focus on ferroptosis and therapeutic vulnerabilities.
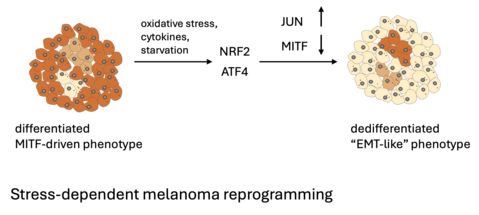
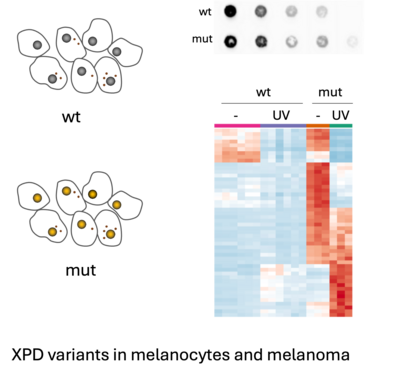
Nucleotide excision repair defects in melanoma
Due to their exposed position in the skin, melanocytes depend on a functional nucleotide excision repair (NER) system to avoid the accumulation of UV-induced mutations. Consequently, persons with certain hereditary variants of the NER protein XPD have a strongly elevated risk of developing melanomas due to the high rate of UV-induced mutations.
Using complementary model systems, we are investigating the role of different XPD variants in the context of melanoma biology and immune therapy. Our aim is to develop a rational basis for therapeutic intervention of melanoma under conditions of NER deficiency.
Prof. Dr. Svenja Meierjohann

Anita Hufnagel
Lena Sorger
Ravi Babu Kollampally
Universität Würzburg
Josef-Schneider-Str. 2, Haus E2
You can find all publications of AG Meierjohann here.
Selected Publications
Freitas FP, Alborzinia H, Dos Santos AF, Nepachalovich P, Pedrera L, Zilka O, Inague A, Klein C, Aroua N, Kaushal K, Kast B, Lorenz SM, Kunz V, Nehring H, Xavier da Silva TN, Chen Z, Atici S, Doll SG, Schaefer EL, Ekpo I, Schmitz W, Horling A, Imming P, Miyamoto S, Wehman AM, Genaro-Mattos TC, Mirnics K, Kumar L, Klein-Seetharaman J, Meierjohann S, Weigand I, Kroiss M, Bornkamm GW, Gomes F, Netto LES, Sathian MB, Konrad DB, Covey DF, Michalke B, Bommert K, Bargou RC, Garcia-Saez A, Pratt DA, Fedorova M, Trumpp A, Conrad M, Friedmann Angeli JP: 7-Dehydrocholesterol is an endogenous suppressor of ferroptosis. Nature. 626:401-410 (2024). doi: 10.1038/s41586-023-06983-9.
Meinert M, Jessen C, Hufnagel A, Kreß JKC, Burnworth M, Däubler T, Gallasch T, Xavier da Silva TN, Dos Santos AF, Ade CP, Schmitz W, Kneitz S, Friedmann Angeli JP, Meierjohann S: Thiol starvation triggers melanoma state switching in an ATF4 and NRF2-dependent manner. Redox Biol. 70:103011 (2024). doi: 10.1016/j.redox.2023.103011.
Kreß JKC, Jessen C, Hufnagel A, Schmitz W, Xavier da Silva TN, Ferreira Dos Santos A, Mosteo L, Goding CR, Friedmann Angeli JP, Meierjohann S: The integrated stress response effector ATF4 is an obligatory metabolic activator of NRF2. Cell Rep 42:112724 (2023). doi: 10.1016/j.celrep.2023.112724.
Jessen C, Kreß JKC, Baluapuri A, Hufnagel A, Schmitz W, Kneitz S, Roth S, Marquardt A, Appenzeller S, Ade CP, Glutsch V, Wobser M, Friedmann-Angeli JP, Mosteo L, Goding CR, Schilling B, Geissinger E, Wolf E, Meierjohann S: The transcription factor NRF2 enhances melanoma malignancy by blocking differentiation and inducing COX2 expressionOncogene 39:6841-6855 (2020). doi: 10.1038/s41388-020-01477-8.
Yu Y, Schleich K, Yue B, Ji S, Lohneis P, Kemper K, Silvis MS, Qutob N, van Rooijen E, Werner-Klein M, Li L, Dhawan D, Meierjohann S, Reimann M, Elkahloun A, Treitschke S, Dörken B, Speck C, Mallette FA, Zon LI, Holmen SL, Peeper DS, Samuels Y, Schmitt CA, Lee S: Targeting the Senescence-Overriding Cooperative Activity of Structurally Unrelated H3K9 Demethylases in MelanomaCancer Cell 33:322-336 (2018). doi: 10.1016/j.ccell.2018.03.009.
Maurus K, Hufnagel A, Geiger F, Graf S, Berking C, Heinemann A, Paschen A, Kneitz S, Stigloher C, Geissinger E, Otto C, Bosserhoff A, Schartl M, Meierjohann S: The AP- 1 transcription factor FOSL1 causes melanocyte reprogramming and transformation. Oncogene 36:5110-5121 (2017). doi: 10.1038/onc.2017.135.
Leikam C, Hufnagel AL, Otto C, Murphy DJ, Mühling B, Kneitz S, Nanda I, Schmid M, Wagner TU, Haferkamp S, Bröcker EB, Schartl M, Meierjohann S: In vitro evidence for senescent multinucleated melanocytes as a source for tumor-initiating cellsCell Death Dis. 6:e17112015. doi: 10.1038/cddis.2015.71.
Haferkamp S, Borst A, Adam C, Becker TM, Motschenbacher S, Windhövel S, Hufnagel AL, Houben R, Meierjohann S: Vemurafenib induces senescence features in melanoma cells. J Invest Dermatol 133: 1601-1609 (2013). doi: 10.1038/jid.2013.6.
Meierjohann S, Kleinschmidt MA, Wende E, Hufnagel A, Wolf K, Friedl P, Gaubatz S, Schartl M: MMP-13 mediates cell cycle progression in melanocytes and melanoma cells: in vitro studies of migration and proliferationMol Cancer 9:201 (2010). doi: 10.1186/1476-4598-9-201.
Leikam C, Hufnagel A, Schartl M, Meierjohann S: Oncogene activation in melanocytes links reactive oxygen to multinucleated phenotype and senescenceOncogene. 27:7070-7082 (2008). doi: 10.1038/onc.2008.323.

